- AIXBIO
- Posts
- AIXBIO Weekly #21 - Nov/27/2023
AIXBIO Weekly #21 - Nov/27/2023

Regulatory Landscape
EU AI Act
Balancing Regulation and Innovation in Foundation Models
EU's AI Act faces changes as France, Germany, Italy push for self-regulation in AI, focusing on application risks and advocating for model cards.
Sanctions are deferred pending systematic code breaches
The European Union's AI Act, a landmark initiative for AI regulation, faces potential modifications under the influence of France, Germany, and Italy. These countries advocate for a more innovation-friendly approach, suggesting self-regulation for AI companies working on foundation models. This proposal includes publishing information about the models and adhering to codes of conduct, initially without penalties for non-compliance.
Foundation models, like GPT-3.5, are at the heart of this debate. These AI systems are trained on vast data sets and are capable of a wide range of tasks, making them both valuable and risky. The U.S. has taken steps to regulate these models, requiring safety tests and reporting, while the U.K. has focused discussions on their risks at the AI Safety Summit.
This two-tier approach to regulation approach is seen as a compromise, balancing the need for oversight with the desire to promote innovation. The pushback against stringent regulation is partly driven by concerns that overregulation could hinder AI development, particularly in Europe. France and Germany, home to prominent AI companies, emphasize the need for a balanced regulation that supports innovation while protecting fundamental rights.
Negotiating the Future of AI: The Spanish Presidency's Role in Shaping EU's AI Law
The AI Act is currently in its final phase, involving crucial negotiations among the EU Commission, Council, and Parliament.
The Spanish presidency is advocating for flexibility in the area of law enforcement within the AI Act. This includes suggesting possible landing zones in the law enforcement area, which is a sensitive aspect of the AI regulation. The negotiations are set to culminate in a political meeting scheduled for December 6, where the final agreement on the AI Act will be sought.
Key aspects of the negotiations include prohibitions on certain AI applications deemed to entail an unacceptable level of risk. The Spanish presidency has proposed accepting bans on practices like untargeted scraping of facial images and emotion recognition in certain contexts. Additionally, there are discussions around law enforcement exceptions, allowing police forces to use AI tools with certain flexibilities.
Another significant aspect of the negotiations is the fundamental rights impact assessment. This new obligation would require users of high-risk systems to conduct an assessment before deployment, focusing on public bodies and private actors providing services of general interest.
Partnerships
AI-Driven Drug Discovery:
Genentech and NVIDIA's Collaborative Leap Forward
The NVIDIA blog post highlights a significant collaboration between Genentech, a biotechnology pioneer, and NVIDIA, a leader in AI technology. This partnership is focused on transforming the discovery and development of new medicines by integrating Genentech’s proprietary algorithms with NVIDIA’s AI supercomputing capabilities.
Central to this collaboration is the use of NVIDIA DGX Cloud, which provides dedicated instances of AI supercomputing and software. This integration is expected to significantly enhance Genentech’s computational drug discovery workflows. Additionally, Genentech plans to utilize NVIDIA BioNeMo, a domain-specific platform that streamlines generative AI applications for computational drug discovery. This platform allows researchers to either pretrain or fine-tune state-of-the-art models, thereby accelerating the drug discovery process.
A key aspect of this partnership is the focus on Genentech’s “lab in a loop” framework. This innovative approach aims to understand complex biomolecular patterns and relationships, which are crucial in disrupting traditional drug development methods.
AI in Dermatology:
Almirall and Absci's Pioneering Partnership for Skin Disease Therapeutics
Absci Corporation announces a groundbreaking partnership with Almirall S.A., a global biopharmaceutical company specializing in medical dermatology. This collaboration marks a significant stride in AI-driven drug discovery, focusing on developing novel treatments for chronic and debilitating dermatological diseases.
The partnership leverages Absci’s proprietary Integrated Drug Creation™ platform, which employs generative AI technology. This innovative approach is set to revolutionize the process of creating and commercializing therapeutic candidates, specifically targeting two dermatological conditions. Absci's platform stands out for its ability to rapidly design and validate de novo therapeutic antibodies, a capability that significantly accelerates the drug discovery process.
Financially, the collaboration is structured to provide Absci with substantial incentives, including upfront fees, R&D, and post-approval milestone payments, potentially totaling up to approximately $650 million across the two programs. This financial arrangement underscores the high expectations and confidence in the partnership's success.
AI and Quantum Simulation Collaboration: SandboxAQ and NVIDIA's Venture into Advanced Drug Discovery and Material Science
SandboxAQ, in collaboration with NVIDIA, is embarking on a groundbreaking venture in AI and quantum simulation. This initiative is set to play a pivotal role in predicting chemical reactions crucial for drug discovery, battery design, and green energy solutions. The collaboration aims to simulate the quantum mechanics fundamental to modern chemistry, biology, and material science.
By harnessing the power of GPU-optimized tensor network methods and NVIDIA's sophisticated software, the collaboration seeks to address complex challenges in science and industry.
A key focus of this venture is the pharmaceutical sector, where the technology will be applied to protein-ligand binding computations, particularly targeting neurodegenerative diseases. This approach is anticipated to bring forth novel solutions for pose and toxicity prediction, as well as the development of next-generation batteries composed of innovative materials.
Innovation
ScienceGPT:
Pioneering AI Research with a Trillion Parameters
ScienceGPT, a one-trillion-parameter generative AI system, at the Argonne National Lab (ALN) in Illinois, USA. This groundbreaking AI model is being trained using data from the Aurora supercomputer, one of the world's most powerful supercomputers, featuring 60,000 Intel GPUs and over 10,000 computing nodes.
The training of ScienceGPT, which is expected to take several months, is currently limited to 256 nodes of the Aurora supercomputer. This limitation is due to the unique design of the Aurora supercomputer, and there are plans to scale up the training process over time. Despite these limitations, the training is a significant step in the development of large-scale AI models.
ScienceGPT is designed to incorporate a wide range of scientific data, including texts, codes, specific scientific results, and papers. This integration aims to speed up scientific research by providing a comprehensive and efficient tool for data processing and analysis. The model operates similarly to ChatGPT but is specifically tailored for scientific applications.
Advancing Biotech with AI:
RoseTTAFoldNA's New Frontier in Protein–Nucleic Acid Structure Prediction\
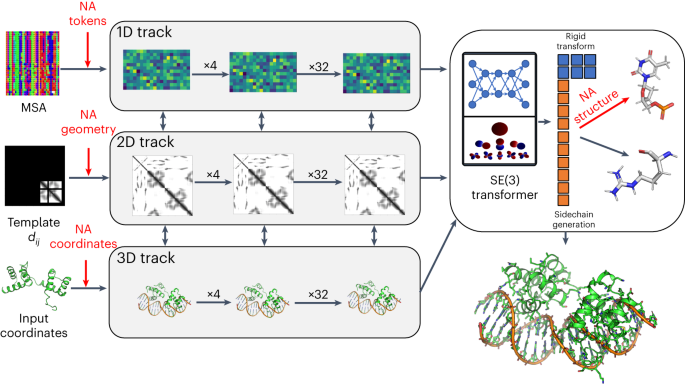
Nature Methods introduces RoseTTAFoldNA, a novel extension of the RoseTTAFold2 platform. This development is pivotal in the realm of biotechnology, specifically for predicting the structures of protein–nucleic acid complexes. The model is a result of integrating data from protein–RNA and protein–DNA complexes, enhancing the predictive capabilities of the AI system.
RoseTTAFoldNA employs a sophisticated three-track architecture. This design is instrumental in refining the representations of biomolecular systems, a critical factor in predicting complex structures. Despite the challenge posed by the limited number of nucleic acid structures available for training, the model exhibits reasonable accuracy. This is particularly noteworthy for structures that lack homologs in the training dataset.
Funding
Advancing Sepsis Diagnosis:
Cytovale's IntelliSep® Secures Major Funding for Nationwide Implementation
Cytovale, a medical diagnostics company, has recently secured $84 million in Series C funding. This significant investment, led by Norwest Venture Partners, will be used to bring their FDA-cleared IntelliSep® test to more U.S. hospitals. IntelliSep® represents a significant advancement in the early detection and treatment of sepsis, a leading cause of death in U.S. hospitals. The test, which delivers results in under 10 minutes from a standard blood draw, is a game-changer for emergency departments (EDs) where rapid, accurate diagnosis is crucial.
Sepsis, often hard to distinguish from other conditions, poses a significant challenge in EDs. Current diagnostic methods are either not specific enough or lack the necessary speed and accuracy. IntelliSep® addresses these issues by utilizing machine learning to analyze immune cell responses, providing a score that indicates the likelihood of sepsis. This innovative approach allows healthcare providers to make more informed decisions quickly, potentially reducing the risk of poor outcomes, including death.
The implementation of IntelliSep® in EDs is already showing promising results. For instance, Our Lady of the Lake Regional Medical Center reported an 80% reduction in all-hands-on-deck alerts for sepsis compared to previous methods. This not only improves patient outcomes but also optimizes the use of hospital resources.
Adoption
Decoding the Adoption Landscape of FDA-Approved Medical AI Devices in the U.S.
The overall utilization of medical AI products is still limited and focused on a few leading procedures. However, utilization has generally increased exponentially for each medical AI procedure.
Analyzing the adoption and usage of over 500 FDA-approved medical AI devices in the United States. The study methodically tracks Current Procedural Terminology (CPT) codes related to medical AI, offering insights into device utilization across different clinical settings. A significant revelation is the limited yet growing utilization of these AI devices, predominantly driven by a few leading devices such as those for coronary artery disease and diabetic retinopathy. This trend is observed despite the widespread approval of AI devices in fields like radiology, neurology, and pathology.
The study highlights the variability in AI algorithm performance across healthcare settings, suggesting that AI's clinical impact is influenced by both technological factors and the complexities of healthcare environments. Economic considerations, including reimbursement models and the costs associated with AI deployment, emerge as crucial determinants of AI device usage. Additionally, the study underscores the role of geographic and economic factors, noting that higher-income, metropolitan areas with academic medical centers are more likely to adopt medical AI technologies.
This comprehensive analysis, based on a database of 11 billion CPT claims, offers a granular view of the AI landscape in medical practice. It calls attention to the challenges of integrating AI into clinical workflows and the need for a multi-faceted approach to AI adoption, encompassing technological, economic, and regulatory aspects. The study concludes by emphasizing the importance of understanding the entire translational pipeline of AI technology, from development to clinical implementation, to ensure its successful and meaningful integration into healthcare systems.
Enhancing Hospital Readmission Predictions: Insights from the PREDICT Trial
The PREDICT trial, conducted by the University of Pennsylvania, represents a significant advancement in the application of AI and ML in biotechnology, specifically in healthcare. The trial's primary goal was to enhance the prediction of hospital readmissions within 30 days, a critical issue in healthcare management. To achieve this, the trial enrolled 500 patients who were monitored for six months using either smartphones or wearable devices after their discharge from the hospital.
The innovative aspect of this trial was the integration of remotely monitored data on physical activity and sleep patterns into a standard prediction model that traditionally used patient demographics, comorbidities, hospital length of stay, and vital signs before discharge. This approach aimed to leverage the rich data available from patient behaviors at home, which is often overlooked in conventional models.
The trial compared the effectiveness of traditional regression methods with nonparametric machine learning regression in predicting readmissions. The results indicated that the machine learning-enhanced models provided more accurate predictions than the standard models. Interestingly, data from smartphones yielded slightly better predictions than data from wearable devices. This finding is particularly relevant considering the widespread use of smartphones, suggesting a scalable approach for remote patient monitoring.
Accelerating Lung Cancer Diagnosis in NHS with AI: A New Era in Medical Imaging
The UK's NHS is set to revolutionize lung cancer diagnosis and treatment by deploying artificial intelligence (AI) tools across 64 trusts in England. This initiative follows the government's commitment of £21 million to integrate AI into the NHS. These advanced tools will primarily analyze X-rays and CT scans, aiming to detect lung cancer more efficiently. Given the high volume of chest X-rays conducted monthly, exceeding 600,000, AI's role becomes crucial in enabling faster and more accurate diagnoses.
The application of AI in the NHS is not new. It has already been implemented successfully in over 90% of England's stroke networks, where it significantly reduced waiting times for treatment. Building on this success, Health and Social Care Secretary Steve Barclay emphasizes the aim to provide timely support to lung cancer patients. Moreover, the government has invested around £123 million to support 86 different AI technologies for various medical purposes, including stroke diagnosis, cardiovascular monitoring, and home-based condition management.
A notable development in this AI initiative is the introduction of AI-Airlock by the Medicines and Healthcare products Regulatory Agency. This regulatory sandbox is designed for AI developers, enabling advanced AI technologies to be utilized in NHS settings before full regulatory approval. This approach is expected to bring early benefits to patients, highlighting the progressive attitude towards AI integration in healthcare.
Navigating the Future of AI in Healthcare: Balancing Promise and Challenges
Duke University School of Medicine and Duke Health are at the forefront of integrating artificial intelligence (AI) into healthcare. Their approach is multifaceted, encompassing efficient surgery scheduling, immediate academic feedback, and accelerated drug discovery. A notable application is the use of AI to predict operating room time, which has proven to be more accurate than traditional methods. This innovation not only optimizes surgical suite usage but also reduces overtime labor costs.
The philosophy guiding these AI applications is to support and enhance human capabilities, not to replace them. For instance, AI algorithms assist schedulers in organizing surgeries more effectively, fostering trust and improving access for patients. Another promising area is the use of AI in medical imaging, such as mammograms, to detect diseases earlier than human analysis alone.
A significant collaboration between Duke Health and Microsoft aims to harness generative AI and cloud technology to transform healthcare. This partnership is exploring the creation of a reference standard for imaging to identify early signs of disease. In the realm of drug discovery, AI is being used to predict interactions between proteins and potential drugs. This approach is based on the similarities between the structure of proteins and human language, allowing for more accurate predictions of protein structures and functions. This method could significantly expedite the drug development process, which traditionally is a lengthy and costly endeavor.
AI to Help Boost NHS Winter Response and Prevent Avoidable Admissions
The National Health Service (NHS) in the UK is adopting artificial intelligence (AI) technologies to enhance its winter response and prevent avoidable hospital admissions. This initiative is part of a broader strategy to utilize tech and data solutions to alleviate the pressures on Accident and Emergency (A&E) departments.
In Somerset, four GP practices are trialing an AI system designed to identify patients with complex health needs who are at risk of hospital admission. This proactive approach enables healthcare professionals to reach out to these individuals for health discussions, potentially preventing the need for hospital visits. Similarly, in Buckinghamshire, AI is being used in conjunction with electronic sensors on household appliances like kettles and fridges. This setup helps monitor changes in patients' eating and drinking habits, alerting healthcare teams to potential health issues.
Birmingham is piloting a different approach, using an algorithm to predict patients at risk of hospital attendances or admissions. This predictive model allows healthcare staff to offer timely interventions, such as social care assessments and medication reviews, to prevent A&E admissions.
These AI-driven initiatives are expected to significantly reduce the number of unnecessary A&E attendances and overnight hospital stays. They also aim to free up a substantial number of GP appointments, easing the overall strain on the healthcare system. The NHS is also expanding its virtual ward program, which facilitates hospital-level care for patients in the comfort of their own homes. This program aligns with the NHS's commitment to leveraging technology for patient-centric care, ensuring comfort and convenience while maintaining high-quality healthcare standards.
Opinions & Future Trends
Exploring Blockchain as a Regulatory Mechanism for AI's Future
The intersection of Artificial Intelligence (AI) and blockchain technology is gaining attention for its potential to enhance data security, transparency, and efficiency. Concerns about AI's control and ethical use are driving the exploration of blockchain as a regulatory mechanism. A survey indicates that nearly half of IT leaders believe this integration could be revolutionary, particularly in data security.
Blockchain's immutable ledgers and smart contracts are seen as potential solutions to ensure responsible AI use, possibly acting as a control mechanism for AI models. However, the scalability and efficiency of decentralized blockchains, especially those handling high volumes of data, pose significant challenges. Hybrid blockchains, which combine elements of private and public chains, are proposed as a solution to these challenges.
Despite the optimism, there is skepticism within the AI community about blockchain's ability to solve the "black box" problem of AI, where decision-making processes are not transparent. Blockchain might indirectly address this by ensuring data integrity in AI training datasets, but it may not make AI's decision-making processes more interpretable.
The potential integration of AI and blockchain is a topic of both optimism and skepticism. It highlights the ongoing efforts to find solutions for responsible AI development and deployment, while also acknowledging the technical and conceptual challenges that lie ahead.
AI and Quantum Tech:
A New Era in Patient Care and Drug Discovery
The integration of artificial intelligence (AI) and quantum technologies (AQ) is poised to bring about a paradigm shift in patient care and the pharmaceutical industry. The current process of developing new pharmaceutical drugs is both time-consuming and expensive, often taking up to 15 years and costing billions. The success rate of these drugs reaching clinical trials is low, primarily due to the high probability of unforeseen toxicities.
Conventional machine learning (ML) methods in drug discovery face challenges like data uncertainty and scalability issues. However, AQ technologies promise to overcome these hurdles by providing deeper insights into how compounds interact with human receptors. This could significantly accelerate the drug development process, potentially reducing it from years to days. These technologies are expected to improve the accuracy of testing, predict drug efficacy and contraindications more precisely, and hasten the transition to human trials.
Beyond drug discovery, AQ technologies have far-reaching applications in medical imaging, diagnostics, and cybersecurity. Quantum sensors, for instance, can detect minute deviations in magnetic fields around vital organs, offering more precise medical imaging. In cybersecurity, the healthcare industry is preparing for quantum-related threats by developing new encryption standards to protect sensitive data.
And ..
United Nations University paper highlights the necessity of data for AI, proposing an International Decade of Data to address challenges and foster global development
The Data Working Group of the Alliance for AI in Healthcare presents a White Paper to explore the crucial questions around fragmentation, technical challenges, frameworks and standards, ethical, legal, regulatory considerations, and cultural and organizational changes that need to be answered in order to bring us closer to a Unified Patient Record.
Stanford University is hosting an AI + Health online conference, bringing together experts from academia, industry, government, and clinical practice to discuss AI's impact in healthcare.
The conference is open to professionals with or without technical expertise and will cover a wide range of topics.